🍿 Starting Page
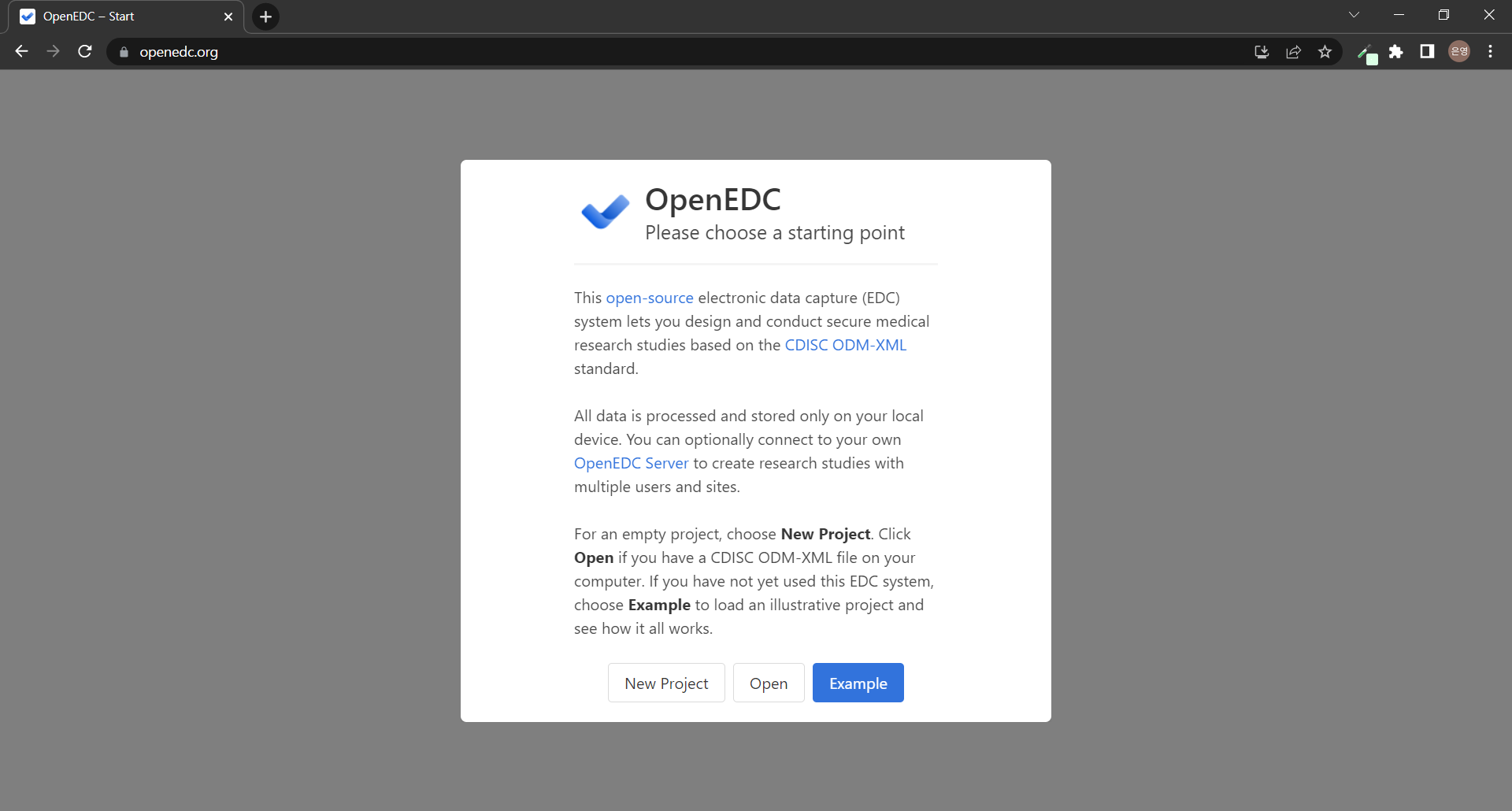
OpenEDC의 시작 페이지는 흰 배경 위의 모달로 시작한다. 굳이..? 스러운 화면 구성이라 왜 이렇게 했는지 찾아보고 큰 문제가 없다면 전체 화면을 사용하면 좋을 것 같다! 모달 안의 내용을 번역해보자면,
"이 EDC(Open-Source Electronic Data Capture) 시스템을 사용하면 CDISC ODM-XML 표준에 기반한 안전한 의료 연구 스터디를 설계하고 수행할 수 있습니다. 모든 데이터는 로컬 장치에만 처리되고 저장됩니다. 선택적으로 사용자 자신의 OpenEDC 서버에 연결하여 여러 사용자 및 사이트로 리서치 스터디를 만들 수 있습니다. 빈 프로젝트의 경우 새 프로젝트를 선택합니다. 컴퓨터에 CDISC ODM-XML 파일이 있는 경우 열기를 누르십시오. 이 EDC 시스템을 아직 사용하지 않은 경우 예를 선택하여 예시 프로젝트를 로드하고 모든 것이 어떻게 작동하는지 확인하십시오."
아직 OpenEDC에 익숙하지 않으므로 예시 프로젝트를 살펴보도록 하자 ~_~
Example 플로우
🍿 Example - Data Collection Mode Page

예시 프로젝트의 경우 Data Collection 모드에서 시작한다. 이미 폼이 설계되어 있어 바로 시험대상자(subejct)들의 데이터를 수집할 수 있도록 한 것 같다. 일단 그럼 설계된 폼을 살펴보기 위해 헤더 우측의 모드 변경 버튼을 이용해 Form Design 모드로 변경하도록 하자!
🍿 Example - Form Design Mode Page

화면 중앙의 디테일 보드와 아래 다섯 가지의 카테고리를 확인할 수 있다. 다섯 가지 카테고리는 각각 Events, Forms, Groups, Items, Choices로 ODM 모델의 구성 요소에 포함되는 것들이다. 여기서 ODM 모델의 구성 요소는 다음과 같다.
The ODM model assumes that a study's clinical data will consist of several kinds of entities. These include subjects, study events, forms, item groups, items, and annotations.
subject는 Data Collection(데이터 수집) 모드에서 추가할 수 있는 것 같고, Events는 저번에 듣기론 주기라고 하시던데 흠.. 자세히는 모르겠지만 일단 방문 주기라고 생각하면 될 것 같다. Forms는 CRF와 유사하다는데..ㅎ 그 부분은 잘 모르겠고 일반적으로 논리적이고 시간적으로 관련된 일련의 정보를 수집한다고 한다. 그리고 이 일련의 Forms는 Events의 하위 구성 요소이다. Groups는 Item Groups를 의미하며 일반적으로 함께 분석되는 밀접하게 관련된 Item들로 구성된다. 그리고 이 Item Groups는 Forms의 하위 구성 요소이다! Items는 혈압 등 개별 임상 데이터 항목을 뜻하고 Item Groups의 하위 구성 요소이다. 마지막으로 Choices는 Item이 선택 항목인 경우 선택지들을 설정하는 기능을 하는 것 같다. 다음은 실제 ODM-XML 표준에 적혀있는 설명이다.
An item is an individual clinical data item, such as a single systolic blood pressure reading. Items are collected together into item groups.
An item group is a closely related set of items that are generally analyzed together. (Item groups are sometimes referred to as "records" and are associated with "panels" or "tables".) Item groups are aggregated into forms.
A form is analogous to a page in a paper CRF book or electronic CRF screen. A form generally collects a set of logically and temporally related information. A series of forms is collected as part of a study event.
A study event is a reusable package of forms usually corresponding to a study data-collection event.
일단 이해하기로는 Events - Forms - Groups - Items 순의 트리 구조인 것 같다. 순서대로 일단 눈에 들어오는 만큼만 살펴보자..!
Events
Events 중 하나를 선택하면 디테일 보드에 Unique ID와 Translated Description이 나타나고 하위 Forms들을 볼 수 있다. Unique ID인 SE.1은 아마 Special Event의 줄임말인 것 같고 Baseline(T0)은 의미를 모르겠어서.. 나중에 설명을 듣고 적어야겠다.

Forms
Forms도 마찬가지로 선택 시 Unique ID와 Translated Description이 나타나고 하위 Item Groups를 볼 수 있다. Baseline Event엔 Basis data(기본 정보) Form과 Medical history(병력) Form이 포함되어 있다.

Item Groups
마찬가지로 Unique ID와 Translated Description을 가지고 Data Type과 Mandatory는 비활성화되어 있다. 기본 정보 Form에는 Personal question Item Group과 Demographic(인구통계학적) questions Item Group이 속해있다. Personal questions Item Group엔 What is your age?, What is your gender? 등 개인 정보를 질문하는 Item들이 속한다.

Item
드디어 Data Type과 Mandatory 드롭다운이 활성화된다! 질문(Item)에 대한 응답의 형식을 지정해줄 수 있다. 응답 데이터 타입으로는 Whole Number, Decimal Number, Yes / No, Text, String, Date, Time, Datetime, Choices, Choices (coded whole number), Choices (coded decimal number), Decimal Number (double)이 있다. Mandatory는 해당 질문(Item)의 필수 응답 여부를 설정한다. 필수로 답해야 하는 질문의 경우 Mandatory를 Yes로 설정해주면 된다!

여자인 경우만 질문에 대답하게, 임신인 경우만 질문에 대답하게 등 조건을 주거나 응답의 범위를 줄 수도 있는데 디테일 보드의 사이드바에서 톱니바퀴 모양(Extended)을 눌러 설정 가능하다! 조건이 있는 경우 Item 앞에 아이콘이 표시된다. Measurement Unit에서 단위를 선택하거나 Calculated 옵션으로 자동으로 계산되게 설정할 수도 있다! Calculated 옵션을 선택했을 경우 more 버튼을 누르면 보이는 Calculation에 원하는 식을 입력하면 된다.


Choices
질문(Item)의 응답 데이터 형식이 Choice(선택)인 경우 해당 질문의 Choices 카테고리의 Add 버튼이 활성화되며 선택지를 추가할 수 있다. 추가한 Choice는 여태까지와 다르게 Unique ID가 아닌 Coded Value를 설정한다. 무엇이 다른 점인진 아직 이해가 가지 않는다... 단순 용어의 차이인가? Data Type과 Mandatory 드롭다운의 경우 다시 비활성화된다.

Subject(시험대상자)들을 조사할 폼을 작성했으니 데이터를 수집하러 가보자! 처음에 봤던 Data Collection 모드로 다시 돌아간다.
🍿 Example - Data Collection Mode Page

새로운 시험대상자(subject)를 추가하려면 식별키를 입력해야하는데.. 아직 형식을 잘 모르겠어서 Project Options의 General Options 탭에서 Subject Key를 자동으로 만들어주는 기능을 활성화해서 시험대상자를 추가해보자!

이렇게 하면 시험대상자의 식별키를 입력하는 빈칸이 사라진다. 밑의 옵션들은 아직 이해가 잘 가지 않는데 일단 Add 버튼으로 시험대상자를 만들어 확인해보자!
1이라는 식별키를 가진 시험대상자가 추가되었고 클릭하면 Options and Audit Trail 버튼이 활성화된다. 버튼을 누르면 다음과 같은 모달이 뜨는데 시험대상자의 식별키와 사이트 정보를 수정하거나 생성일을 확인하거나 시험대상자 정보를 삭제할 수 있는 것 같다!

시험대상자 1이 되어 예시로 만들어진 폼에 대한 응답을 해보자!

ㅇ0ㅇ BMI 질문의 Measurenet Unit을 Calculated로 하고 Calculation에 Weight / Height ^ 2로 해놨더니 입력 칸이 비활성화되어 있는 대신, 키와 몸무게를 입력하면 자동으로 BMI 지수가 계산되어 입력된다!
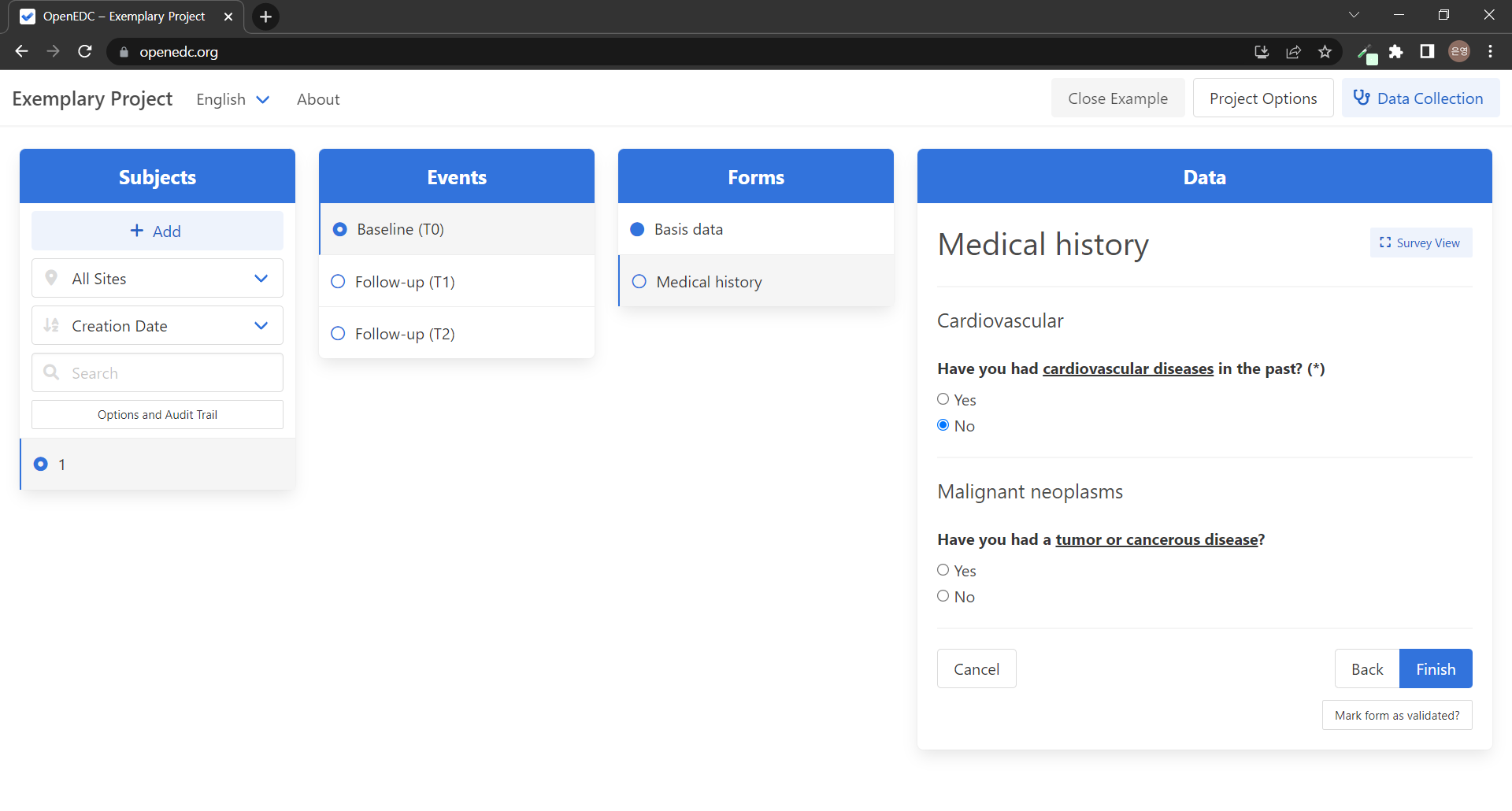
Mandatory를 Yes로 설정해둔 질문의 경우, 질문 맨 마지막에 (*) 표시가 붙으며 응답하지 않고 Finish 버튼을 누를 경우 필수 응답 마크가 표시된 질문에 답해달라는 경고 문구가 뜬다!

폼의 응답을 마친 경우 왼쪽의 파란 동그라미가 채워지게 된다. 부가적으로 질문에 커서를 올리면 우측에 시계 아이콘이 뜨는데 이 아이콘을 클릭하면 응답을 제출한 시간과 사용자, 응답 값의 히스토리를 볼 수 있다. 폼 제목 우측 Survey View 버튼을 클릭하면 설문조사 폼을 전체 화면으로 볼 수 있고 Finish 버튼 하단 Mark form as validated? 버튼을 누르면 해당 응답 폼을 인증된 것으로 표시해둘 수 있다.
이제 Report View 모드로 가기 전에, 다양한 응답을 받고 그래프로 확인할 수 있도록 랜덤한 시험대상자 100명을 추가하도록 하자! Project Options의 General Options 탭에서 Example Data를 추가할 수 있다. 시간이 좀 걸린당..

🍿 Example - Report View Mode Page

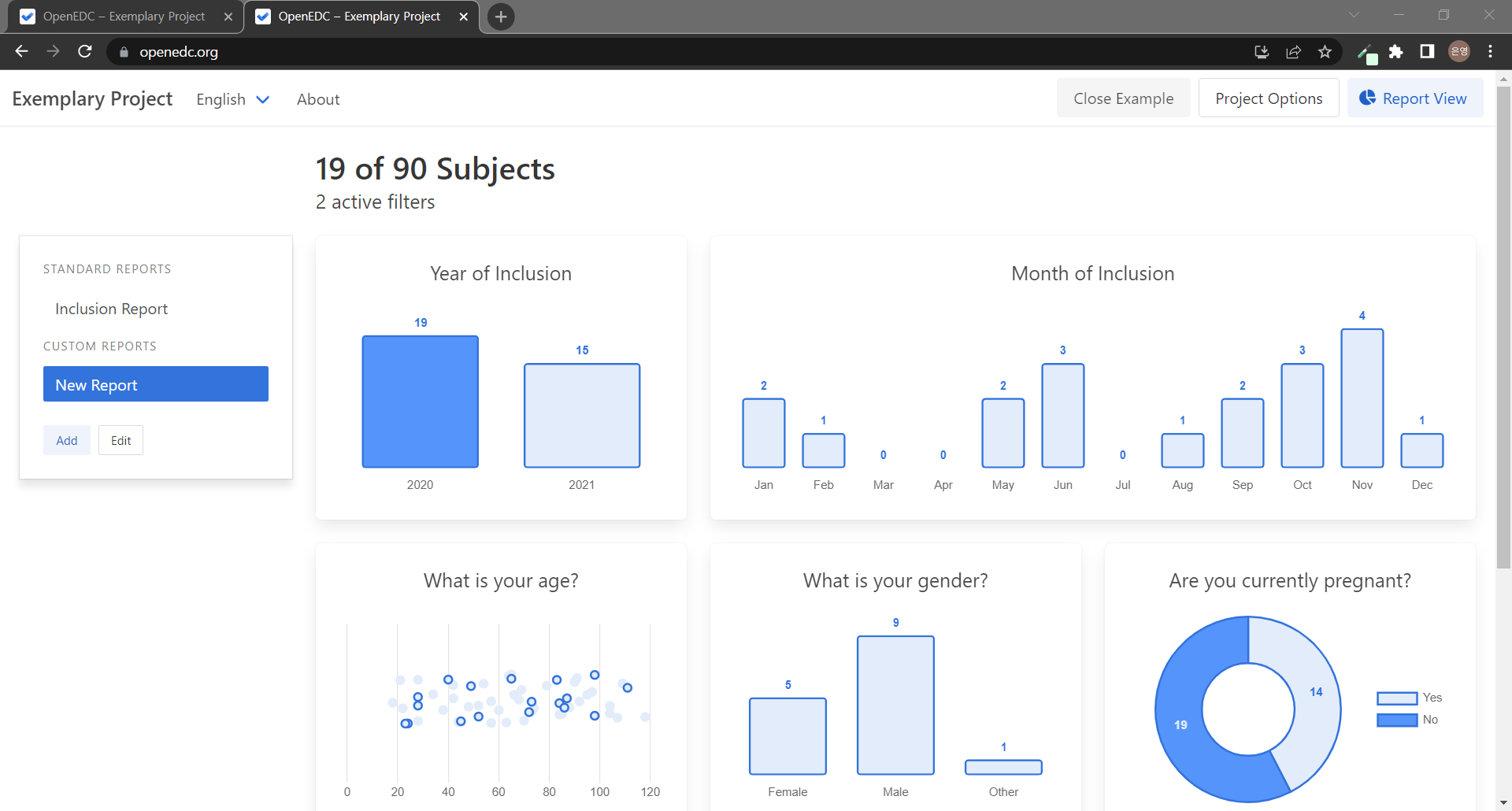
처음 Report View 페이지에 들어오면 시험대상자(subject) 수가 상단에 적혀있고 표준 보고서인 Inclusion Report 그래프들을 볼 수 있다. 시험대상자들의 Site, Year of inclusion, Month of Inclusion들을 확인할 수 있는데 지금 우리 시험대상자들의 경우 모두 No Site이고 2022년 5월에 만들어졌으므로 모두 같은 항목 안에 들어가게 된다..! 질문에 따른 다양한 그래프들을 보기 위해 새로운 보고서를 만들어보자! 기본적으로 추가되어있는 New Report를 사용해도 되고, Add 버튼으로 새로운 보고서를 만들 수도 있다.
New Report로 이동해 + 버튼을 누르면 폼의 질문을 선택해 그에 대한 그래프를 만들 수 있다. New Report를 클릭했을 때 뜨는 Edit 버튼으로 보고서의 이름이나 필터를 수정할 수도 있고 보고서를 삭제할 수도 있다.

Bar Chart, Pie Chart, Donut Chart / Scatter Chart 중 응답 유형에 따라 원하는 차트를 선택할 수 있으며 차트의 크기 또한 선택할 수 있다!

그래프의 원하는 영역을 선택해 필터를 활성화시킬 수도 있다.
🐱👤 참고 :
https://www.cdisc.org/standards/foundational/odm-xml/odm-xml-v1-3-2
'프로젝트 > OpenEDC' 카테고리의 다른 글
| [OpenEDC] 중간 회고회고 (0) | 2022.06.20 |
|---|---|
| [OpenEDC] 스킨 커스텀하기 - 2 (0) | 2022.06.13 |
| [OpenEDC] 스킨 커스텀하기 - 1 (0) | 2022.06.10 |
| [OpenEDC] 다크모드 지원 (0) | 2022.05.31 |
| [OpenEDC] 한국어 추가하기 (0) | 2022.05.27 |



